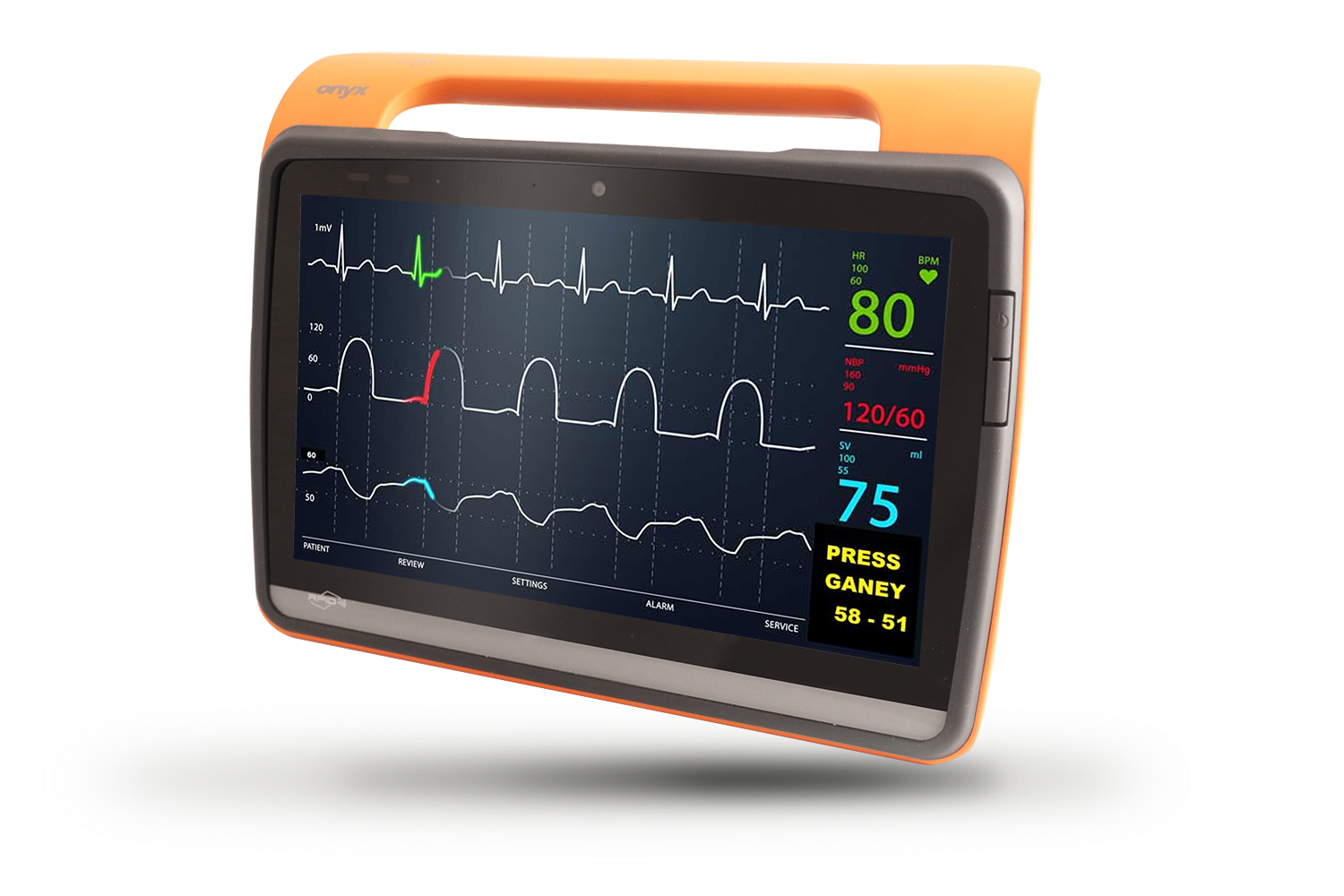
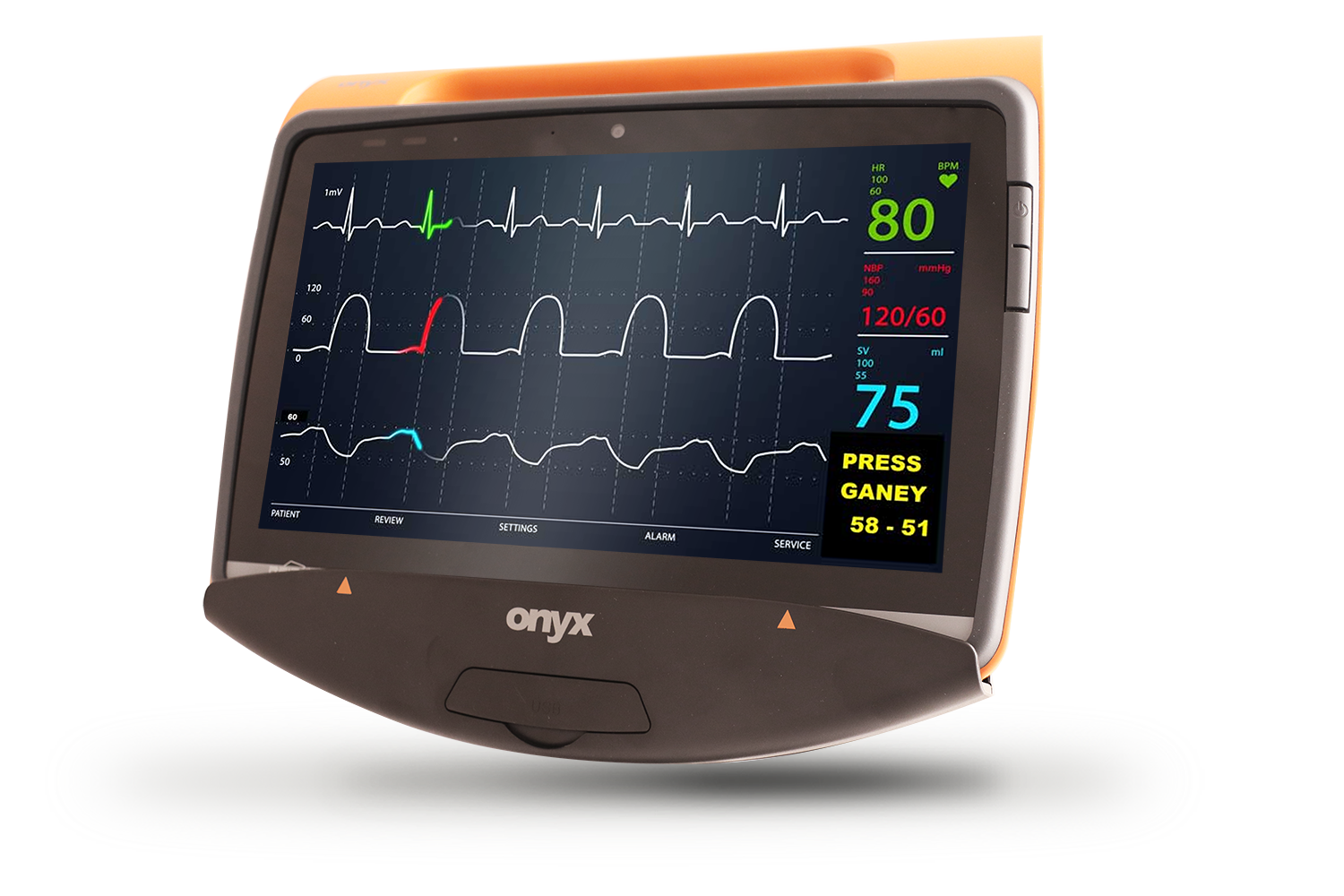
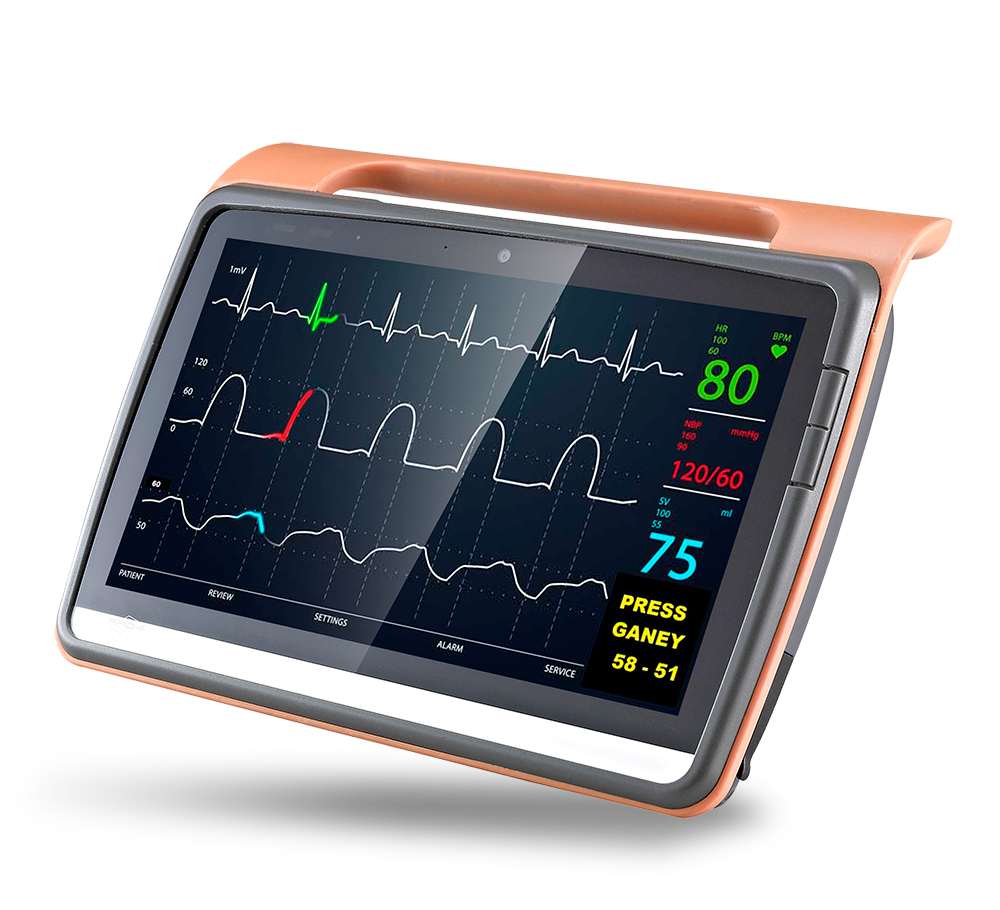
MD116I
12”Fanless Intel ATOM / i5 Medical Tablet
Product Feature

- Medical Tablet PC
- Intel® Apollo Lake processor / Kaby lake U processor
- Dual hot-swappable batteries for 24/7 operation
- Dual smart card readers for multiple-care workflow
- Standard USB Type-A and RJ45 for easy connection to medical devices
- IP54 certified & 3 feet drop resistant
- ORION client compatible
- Instant vehicle docking system
- Automotive Directive and EN1789 compliance for ambulances/medical transportation vehicles
Specifications
| Main Specifications | |
| Processor | Intel® Kaby Lake Core i5 U Processor |
| System Memory |
DDR4 SO-DIMM x1, Default 4 GB (Up to 16 GB) |
| OS Support | Microsoft® Windows 10 IOT LTSB |
| Storage | M.2 Interface x1, Default 64 GB |
| Wireless Communication | 802.11 a/b/g/n + BT4.0 (Optional) |
| Camera | 2 MP (Front) / 5 MP (Rear) (optional) |
| Touchscreen | Projected Capacitive Touchscreen,w/Gorilla Glass |
| Digital Accelerometer | ADI ADXL345 |
| Ambient Light Sensor | TAOS TSL2581 |
| LTE+GPS | Sierra EM7421 x 1(Optional) |
| Function Keys | x2 |
| LED Indicators | x2 |
| Speaker | 2W x1 |
| Internal Mic | x1 |
| Power Requirement | DC 12V |
| Security | Smart Card Reader x2 , RFID x1 (optional) |
| Display | |
| Display Size | 11.6” 16:9 Widescreen LED Panel |
| Resolution | FHD 1920 x 1080 |
| Max. Colors | 16.7M |
| Contrast Ratio | 800:1 |
| Luminance (cd/m2) | 600nits |
| I/O | |
| USB | USB 3.0 (Type-A) x1, USB 2.0 (Type-A) x1 |
| Ethernet | Gigabit LAN x1 |
| DC-In | DC-In Jack x1 |
| Docking | Docking Connector x1 |
| Battery Pack | |
| Battery Type | Li-ion |
| Battery Capacity | 4545 mAh (32.72Wh) x2 |
| Battery Run Time | Approx. 3.5 hrs |
| Mechanical and Environmental | |
| VESA | VESA 75 via docking station |
| Degree of Protection | IP54 Whole unit (IP65 in the front) |
| Operating Temperature | 0°C ~ 35°C (32°F ~ 95°F) |
| Storage Temperature | -20°C ~ 60°C (-4°F ~ 140°F) |
| Storage Humidity | 10%~95%@35°C, non-condensing |
| Dimensions | 312 (W) x 239 (H) x 37 (D) mm |
| Packing Size | 400 x 345 x160 mm |
| Gross Weight | 3 kg (6.6 lb) |
| Net Weight | Approx. 1.45~1.8 kg |
| Certifications |
CE: EN 60601-1-2:2020 (EMC4.1), UL: ANSI/AAMI ES60601-1:2005 & A1:2012 & A2:2021 (V3.1) cUL: CAN/CSA-C22.2 No. 60601-1:2014 (V3.1) |
| Docking Station | |
| Power | DC 12V |
| Battery | 4545 mAh (32.72Wh) x2 |
| USB | USB 3.0 (Type-A) x1, USB 2.0 (Type-A) x1 |
| Ethernet | Gigabit LAN x1 |















.jpg)